Background Venous thromboembolism (VTE) is a frequent complication in patients with cancer and can be provoked by the tumor or treatment.An imbalance between coagulation factors and anticoagulant proteinshas been associated with enhanced VTE risk in these patients. Particularly, elevated fibrinogen, FVIII and von Willebrand factor and decreased antithrombin, increase the risk of cancer-associated VTE.
Aim To investigate whether artificial intelligence methodologies based on input of patient characteristics and coagulation parameters can predict VTE in patients with cancer.
Methods In the ongoing study, all-type of cancer patients with or without previous VTE are enrolledand thrombotic events were followed up over a period of 2 years or until death. Demographics, cancer type and treatment, medications, and VTE events before and during the study were documented. Coagulation factors, antithrombin, fibrinogen, FVIII and Von Willebrand Factor were determined in all samples. The artificial intelligence method neural networks was used to develop a prediction model for VTE in patients with cancer. Final input parameters were age, sex, prior VTE, use of anticoagulants and statins, fibrinogen, antithrombin, FVIII and von Willebrand factor.
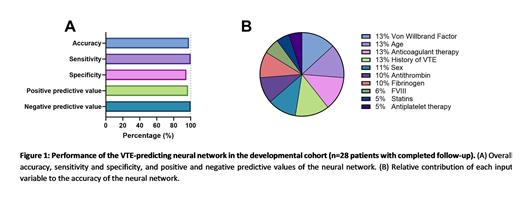
Results To date, 118 patients were enrolled in the study, and 28 patients completed the follow-up (24%). A neural network was developed in the sub-cohort of patients that had completed the follow-up period (n=28), of which 7 patients had a VTE during the follow-up period. The top-performing neural network was selected. The developed neural network had an overall accuracy of 98%, a sensitivity of 100% and a specificity of 95%. Furthermore, the negative predictive value was 100% and the positive predictive value was 97% (Figure 1A). The most important variables in the neural network were Von Willebrand Factor, age, anticoagulant therapy and history of VTE, contributing 13% each. Sex, antithrombin and fibrinogen contributed 11%, 10% and 10%, respectively, whereas FVIII (6%) , statin use (5%) and antiplatelet therapy (5%) had a minor contribution (Figure 1B).
We validated the neural network in the “on-study” sub-cohort, consisting of patients that did not yet complete the follow-up period. Until now, 2 VTE events were recorded in this group; other patients in the sub-cohort remain potentially at risk for VTE. The neural network was able to accurately discern the patients that experienced a VTE during follow-up, resulting in a sensitivity of 100%, and a negative predictive value of 100%. Moreover, the neural network indicated an increased risk of VTE in 37 subjects out of 88 “on-study” patients. The neural network scores the risk of VTE as a value ranging from 0 (no risk) to 1 (high risk). We stratified the neural network VTE risk estimate according to the Khorana and PROTECHT scores for each patient and found a positive association between both the Khorana and PROTECT scores and the neural network VTE estimate (p<0.05).
Conclusions A neural network based on patient characteristics, coagulation parameters, and medication use predicts VTE in our cohort of cancer patients. Although the initial validation of the neural network is promising, further clinically validation is needed.
Disclosures
De Laat-Kremers:Synapse Research Institute: Current Employment, Other: Synapse Research Institute is part of the Diagnostica Stago group.. De Laat:Synapse Research Institute: Current Employment, Other: Synapse Research Institute is part of the Diagnostica Stago Group.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal